Home
Welcome to Williams Research Group website. These pages provide some introduction to our latest news, research interests, academic publications, group members, outreach and engagement activities and information on how to apply to join us.
Our research focusses on sustainable chemistry, carbon dioxide utilization, catalysis, polymer chemistry, materials, inorganic/coordination and organometallic chemistry. We are a diverse, multi-national and multi-disciplinary team whose objective is to provide innovative solutions to major global and environmental challenges. We work in modern synthesis laboratories in the chemistry research laboratory and have specialist laboratories equipped to scale and monitor polymerizations, process and understand polymer properties for a wide range of applications. Our polymers are used as plastics, elastics, adhesives, surfactants, coatings, in solid state batteries, consumer products, electronics and many more. All our polymers are designed to be renewably sourced, show excellent properties for their target application and to be fully recyclable after use. We work with many different academic partners to solve these problems across fields, including engineering, materials science, computing, environmental economics, policy and law. We also often work in partnership with industries, businesses, charities and with individuals motivated by sustainability challenges. Our team regularly undertaken outreach and engagement activities, including Charlotte's work with professional societies to provide future technology policy briefings.

Recent News
- Job Alert: The Williams group is recruiting a PDRA in Sustainable Polymer Chemistry to join the team. The closing date for applications is 15th April 2024. For more information and to apply, please click here.
- February 2024: iCAST Creative Hub Launch Event
- Professor Charlotte Williams presented on the vision for innovation and co-creation of projects with industry. Dr Ryan Kerr and Dr Greg Sulley also attended the event which was held at Brunel’s Carriage Works, Swindon.

- ‘Cleaning up cleaning: policy and stakeholder interventions to put household formulations on a pathway to net zero’
-
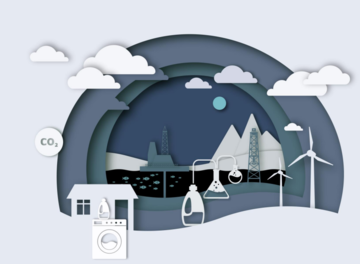
Researchers from the group, along with a team in environmental economics at the Oxford Smith School and working with Unilever Home Care have jointly reported on the policy interventions required to improve sustainability of household products. Their aim is to reduce the consumption of fossil-fuel derived feedstocks used in many chemical formulations ubiquitous in everyday household products.
-
- ‘Designing a circular carbon and plastics economy for a sustainable future’ – Perspective in Nature published
- A collaborative team from the Williams group, as well as other teams in law and environmental economics, published a perspective piece in Nature today detailing a set of ambitious targets to achieve a net zero plastic economy. Their report analyses the current plastic economy and several proposed scenarios to combat the multifaceted issues with current plastics manufacturing and use. They find that only the most drastic of measures will be sufficient to achieve substantial change by 2050. Their proposal for the future of the plastics economy centres on: a reduction on plastic consumption by half, replacement of all fossil-fuel-based plastics with renewable feedstocks, designing materials to enable 95 % of all plastics to be recycled, and minimizing environmental impacts by removing pollution and hazards to all organisms and decreasing the carbon footprint in their lifecycle.
- The team have been advising the UN Environment Programme and UK Government to aid with the ongoing discussions around the second global pollution treaty, due for completion in 2024, focussing on the lifecycle of plastics.
- The full article can be found here and this work is highlighted on the departmental website.

- January 2024: Collège de France and the Royal Society Building a Climate Resilient Society
- Charlotte presented on carbon dioxide transformation into chemicals at the bilateral discussion meeting between the Royal Society and Collège de France. The meeting focussed on key challenges we need to solve to improve climate resilience in society.

- Sustainable Carbon for a Net Zero Chemical Industry Conference
- Register here for the ‘Sustainable Carbon for a Net Zero Chemical Industry’ one-day conference at the University of Liverpool. This event is supported by the EPSRC Cleaner Futures Prosperity Partnership and will include talks from industry and academia on solutions to realise net zero. It promises to be a great chance to network and build collaborations!

- Group Christmas Lunch 2023
- The group celebrated the year with our Christmas lunch and secret Santa gift exchange. 2023 was a great year for the group with lots of exciting research developments - thank you all for your hard work!

- Congratulations to Ryan Kerr for winning the poster prize at the ‘Chemical Feedstocks for Sustainable Industry’ conference. This was attended by industry and academia focusing on Sustainable Chemicals from Biomass Residues, End-of-Life Plastic Recycling, and Oleochemicals – December 2023
- Ryan’s poster was titled ‘The Synthesis, Mechanistic and Property Evaluation of Poly(ester-alt-ethers) from Commercial Monomers and a Zr(IV) Catalyst’.

-
December 2023: Royal Society Discussion Meeting Green Carbon For the Chemicals Industry of the Future
-
Charlotte Williams, Richard Catlow, Matthew Rosseinsky, Matthew Davidson and Graham Hutchings organized a 2-day scientific discussion meeting focussed on Green Carbon for the Chemicals Industry of the Future. It was an inspiring 2 days with lectures from international leaders in academia and industry.

-
-
December 2023: Society of Chemical Industries Annual Review Meeting
-
Charlotte presented our work in carbon dioxide activation and polymers at the SCI Annual Review meeting in London. It was inspiring to see the breadth of organic chemistry being presented by the early career researchers.

-
-
EPSRC IAA Case Study - Sustainably sourced, degradable polymers for homecare application
-
The MPLS Division highlight a collaboration from the group. Read more here.
-
- Women in Chemistry Research Symposium – Oxford, November 2023
- Prof. Charlotte Williams and Prof. Fernanda Duarte gave lectures on their research, alongside 8 short talks from DPhil and Postdoctoral researchers in the Department of Chemistry. The afternoon of talks was followed by a poster session where DPhil student Francesca Fiorentini presented her work on ‘Understanding Catalytic Synergy in Dinuclear Polymerization Catalysts for Sustainable Polymers’.
- Chemical Science Symposium: Chemistry of Polymers, October 2023
- Last week Charlotte, Katharina, Sandy, Thom and Kam attended the Chemical Science Symposium: Chemistry of Polymers at the Royal Society of Chemistry in London. Charlotte gave a keynote presentation on Oxygenated Polymers for Solid State Batteries. Katharina, Sandy, Thom and Kam all presented posters on their work on sustainable polymers.

- October 2023: American Chemical Society Sustainable Polymers, Safety Harbor, USA
- Charlotte gave a lecture on polyester thermoplastic elastomers at the meeting. It was excellent to celebrate 10 years of the conference and discussion forum – our team have attended every meeting. Marc Hillmyer has done an amazing job as conference chair and the future looks set to be equally strong in the hands of the new chair Thomas Epps III.

- October 2023: Charlotte Williams Awarded the Boulder Scientific Lectureship of Colorado State University
- Charlotte has been awarded the 2023 Boulder Scientific Lectureship and last week visited Colorado State University to present her lecture and meet the scientists and students. It was a great visit – here she is collecting her award from Eugene Chen – in addition to the useful discussions with the staff and students in the department, she also met with scientists at with the Boulder Scientific Company and with scientists from NREL. It was also an excellent opportunity to catch up with former group members Dr Wilf Diment and Dr Gloria Rosetto.

- Congratulations to Freya Butler for winning the top Inorganic Part II Examination Prize! We are thrilled for this wonderful recognition of Freya's hard work!

- September 2023: European Chemical Society Conference in Salerno
- Charlotte gave a plenary lecture at the EuChemS Conference Green and Sustainable Chemistry in Salerno.

- Professor Williams will be speaking at Thieme WebCheminar 2023. For more information please visit: https://events.thieme.com/webcheminar/

-
Professor Williams will be speaking at IUPAC2023 from 20th - 25th August 2023 in the Hague, Netherlands. To register, please visit: iupac2023.org

- July 2023: Gordon Research Conference Polymer Recycling and Upcycling, New Hampshire, USA
- Charlotte presented a lecture on catalysts for polycarbonate and polyester recycling. The meeting was focussed on viable interdisciplinary solutions for plastics recycling and redesign. Thom McGuire and Madeleine Smith both gave posters at the conference. It was a great scientific meeting with very lively poster sessions.

- May 2023: Gordon Conference (GRC) Carbon Capture, Utilization and Storage, Les Diablerets, Switzerland
- Charlotte presented a lecture on carbon dioxide utilization at the GRC. It was a very broad-ranging scientific meeting, tackling a major problem. The location was very beautiful – an opportunity to visit the glacier.

- February 2023: RSC Tilden Prize Award Symposium, Durham
- Charlotte was awarded the RSC Tilden Medal at the University of Durham, with the medal being presented by Judith Howard FRS. Russell Taylor organised an afternoon symposium on sustainable chemistry with invited lectures from Dr Laurie King (Manchester Metropolitan) and Dr Stephen Poulson (Johnson Matthey). It was a very enjoyable visit and fun afternoon of science.

-
Congratulations to Madeleine Smith for winning a poster prize at the 9th UK Catalysis Conference for her poster on 'Heterodinuclear Mg(II)M(II) (M = Mn, Fe, Ni, Cu and Zn) Catalysts for the Chemical recycling of Poly(Cyclohexene Carbonate)'!
-
Holly Yeo speaks to Oxford Chemistry Alumni Reception, January 2023
DPhil student Holly Yeo spoke to 80 Oxford Chemistry alumni during an outreach event at The Royal Institution, London. Holly presented our group’s work in green chemistry and her DPhil project on polymer electrolytes.

- Professor Williams will be speaking at this year's Sustainable Chemistry Online-Symposium on 1st December 2022. Come along to hear more about our group's work! For more information and registration, please visit: https://en.gdch.de/network-structures/divisions/sustainable-chemistry/events.html

- Congratulations to Georgina Gregory for winning an oral prize at RAPS 2022 for her talk on polymer electrolytes for Li-ion batteries!

-
Congratulations to Professor Charlotte Williams for receiving the The Royal Society Leverhulme Medal (2022)
Charlotte has been awarded the Royal Society Leverhulme Medal for pioneering work developing and understanding high performance carbon dioxide utilization catalysts and for the chemistry of next-generation plastics. The award recognises our team’s research in polymerization catalysis, mechanisms and sustainable polymer chemistry. The Leverhulme Medal is awarded by the Royal Society triennially for outstanding contributions in chemical engineering and applied chemistry. https://royalsociety.org/grants-schemes-awards/awards/leverhulme-medal/

- Congratulations to DPhil student Francesca Fiorentini for winning a poster prize at the Liverpool Catalysis Summer School
- July 2022
- July 2022
-
Well done to Part II students Tatiana Foggit and Sevven Smith for completing their thesis and viva! All the best for the future.
-
June 2022

-
- Congratulations to Kam Poon and Dr Greg Sulley for each winning a poster prize at the BPC2022 conference in Bordeaux!
-
June 2022


-
- Congratulations to Dr Lisa Häfele and Dr Gloria Rosetto for completing their PhDs!
- May 2022


- May 2022
- Lindau Meeting 2022
- Kat, first year DPhil student in the group, has been selected by the German Academic Scholarship Foundation to participate in the 71st Lindau Nobel Laureate Meeting.
- Nature News Feature on Carbon Upcycling
- Charlotte Williams discusses, with other experts, the opportunities and challenges associated with using carbon dioxide to make fuels, polymers, concrete and more products. The Nature news article, written by Mark Peplow, was published 29 March 2022. https://www.nature.com/articles/d41586-022-00807-y

- Charlotte Williams discusses, with other experts, the opportunities and challenges associated with using carbon dioxide to make fuels, polymers, concrete and more products. The Nature news article, written by Mark Peplow, was published 29 March 2022. https://www.nature.com/articles/d41586-022-00807-y
- Congratulations to Georgina Gregory for the award of a Junior Research Fellowship in Chemistry at Wadham College
- Georgina has been awarded a JRF in Chemistry at Wadham to pursue her research into oxygenated polymer electrolytes for use in lithium ion batteries. George is leading a team working across the departments of Chemistry, Materials and Engineering investigating the synthesis, characterization and testing of the new polymers. In addition to her JRF, she is also supported by the Faraday institution project SOLBAT where she is a research theme leader (composite cathode).

- Georgina has been awarded a JRF in Chemistry at Wadham to pursue her research into oxygenated polymer electrolytes for use in lithium ion batteries. George is leading a team working across the departments of Chemistry, Materials and Engineering investigating the synthesis, characterization and testing of the new polymers. In addition to her JRF, she is also supported by the Faraday institution project SOLBAT where she is a research theme leader (composite cathode).
-
Congrats to Gloria Rosetto and Wilf Diment for each winning a poster prize at the EYCheM Conference, hosted virtually by the University of Fribourg (CH).
-
January 2022
-
- Congratulations to Dr Said Said, Dr Greg Sulley, and Dr Wilf Diment for completing their PhD Vivas!
- November 2021

- November 2021
- Charlotte Williams Presents at COP26
- Last week Charlotte attended COP26 and presented on our work on carbon dioxide utilization. Her presentation was in the UK Pavilion as part of Science and Innovation Day on ‘Innovation and Diversity’. Charlotte joined other women from across the world to discuss the opportunities and challenges in this area. She highlighted the opportunity for innovation to create methods to add value to carbon dioxide wastes and to transform them into useful products, like polymers. She also reflected on the challenges of achieving greater diversity in academic chemistry as well as in entrepreneurship. You can see the panel discussion here: www.youtube.com/watch?v=CJHlVHKc3tg

- Last week Charlotte attended COP26 and presented on our work on carbon dioxide utilization. Her presentation was in the UK Pavilion as part of Science and Innovation Day on ‘Innovation and Diversity’. Charlotte joined other women from across the world to discuss the opportunities and challenges in this area. She highlighted the opportunity for innovation to create methods to add value to carbon dioxide wastes and to transform them into useful products, like polymers. She also reflected on the challenges of achieving greater diversity in academic chemistry as well as in entrepreneurship. You can see the panel discussion here: www.youtube.com/watch?v=CJHlVHKc3tg
- Williams Group Polymers Feature in the London Design Museum Exhibition ‘Waste Age’
- Our work is currently featured at The Design Museum London in the exhibition “Waste Age: What can design do?”. Our exhibit demonstrates how polymers can be made from waste CO2 and citrus peel (limonene oxide). These polymers could be useful contributors to a future circular economy where our waste can be utilised to make new, high performance materials. In an accompanying film, Prof. Charlotte Williams discusses how our chemistry might contribute to a sustainable future for plastics. The exhibition is open until 20 February 2022.

We thank the London Design Museum Felix Speller for the photograph

- Our work is currently featured at The Design Museum London in the exhibition “Waste Age: What can design do?”. Our exhibit demonstrates how polymers can be made from waste CO2 and citrus peel (limonene oxide). These polymers could be useful contributors to a future circular economy where our waste can be utilised to make new, high performance materials. In an accompanying film, Prof. Charlotte Williams discusses how our chemistry might contribute to a sustainable future for plastics. The exhibition is open until 20 February 2022.
-
Well done Wilf for winning a poster prize at the AGICHEM 2021 conference, hosted virtually by Imperial College London.
-
September 2021

-
- Congratulations to Part II student Matylda for successfully completing and handing in her thesis. She has done exceptionally well during these difficult times -- we wish you the best for the future!
- June 2021

- June 2021
- Charlotte Williams elected as a Fellow of the Royal Society, May 2021
- Charlotte has been elected as one of 60 new fellows of the Royal Society in 2021. The Royal Society is a Fellowship of many of the world's most eminent scientists, living and working in the UK and the Commonwealth, and is the oldest scientific academy in continuous existence. Each member has been selected for their outstanding contributions to their scientific field. Professor Charlotte Williams OBE becomes a Fellow for her work in developing new sustainable technologies for polymer production and carbon dioxide usage. She aims to combine unconventional raw materials with implementable materials production, processing and to provide polymers designed for recycling and, ultimately, complete degradation. More information can be found on her fellows page: https://royalsociety.org/people/charlotte-williams-10869/

- Charlotte has been elected as one of 60 new fellows of the Royal Society in 2021. The Royal Society is a Fellowship of many of the world's most eminent scientists, living and working in the UK and the Commonwealth, and is the oldest scientific academy in continuous existence. Each member has been selected for their outstanding contributions to their scientific field. Professor Charlotte Williams OBE becomes a Fellow for her work in developing new sustainable technologies for polymer production and carbon dioxide usage. She aims to combine unconventional raw materials with implementable materials production, processing and to provide polymers designed for recycling and, ultimately, complete degradation. More information can be found on her fellows page: https://royalsociety.org/people/charlotte-williams-10869/
- Hot Paper Angew. Chem. Int. Ed, May 2021
-
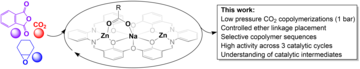
Congratulations to Alex for an Angewandte Chemie 'hot paper' for his work on heterotrimetallic catalysts for carbon dioxide copolymerization and switchable catalysis Alex's paper describes a series of new heterometallic catalysts featuring Na(I) and Zn(II) ions which show efficient catalysis for carbon dioxide/epoxide ROCOP. The catalysts allow for the selective placement of ether linkages in the polymer chains and particularly at chain ends, which serves to stabilise the resulting PCHC. The paper includes detailed kinetic investigations which shed light on the catalysts unusual selectivity and allow for understanding of their performances in switchable polymerizations using epoxide, carbon dioxide and anhydride monomers. It also includes a structure-activity study using other metals which underscores the unique and important role for Na(I) in the best performing catalysts.
-
- UK Catalysis Hub PhD Student Webinar, April 2021
- Greg Sulley gave a virtual talk for the UK Catalysis PhD Student Webinar Series, titled "Switchable Catalysis for the Preparation of CO2-Derived Polymers: The Case of a Heterodinuclear Zn(II)/Mg(II) Organometallic Catalyst". The talk was recorded and can be found here: https://ukcatalysishub.co.uk/uk-catalysis-hub-phd-students-webinar-on-ca...
- Unilever Clean Future Brilliance Award 2021
- Profs. Williams (Oxford), Rosseinsky (Liverpool) and Cooper (Liverpool) have been jointly award a Unilever Clean Future Supplier’s Brilliance Award 2021. The award, given for the first time to an academic team, recognises the individuals work unlocking new areas of materials science such as breakthrough catalysts transforming carbon dioxide to chemicals.

- Profs. Williams (Oxford), Rosseinsky (Liverpool) and Cooper (Liverpool) have been jointly award a Unilever Clean Future Supplier’s Brilliance Award 2021. The award, given for the first time to an academic team, recognises the individuals work unlocking new areas of materials science such as breakthrough catalysts transforming carbon dioxide to chemicals.
- Dr Fernando Vidal Pena awarded a Marie Skłodowska-Curie Actions Fellowshi, February 2021
- Congratulations to Fer on being awarded the MSCA Fellowship, to make Poly-SO2 to explore new directions and materials in ring-opening copolymerization.

- Congratulations to Fer on being awarded the MSCA Fellowship, to make Poly-SO2 to explore new directions and materials in ring-opening copolymerization.
-
Super Science Saturdays, Oxford University Natural History Museum
-
Our work featured on Saturday 28 November 2020 the Oxford University Museum of Natural History Super Saturdays Exhibit ‘Diversity in Plastics’. Our interactive and virtual exhibit uses a polymer lifecycle to stimulate engagement, discussion and exploration through a series of tasks to our approach to improve sustainability across the life cycle. It also uses this cycle to introduce some of the Williams team and explain what motivates them and what might happen in a typical day doing research.

-
- Congratulations Dr Natalia Reis on completing her PhD viva, October 2020

- Congratulations Dr Fernando Pena for being awarded a Newton International Fellowship 2020 sponsored by the Royal Society! Fer aims to broaden the scope of CO2 copolymerisation to make sustainable polymers with improved mechanical/physical properties and implement new routes of chemical degradation and depolymerisation.

- Congratulations Professor Charlotte Williams for being recognised by the Queen of England as an Officer for the Order of the British Empire (OBE) for outstanding services in Chemistry
- October 2020
- Dr Alex Plajer awarded Royal Commission of 1851 Research Fellowship
- Congratulations to Alex who has been awarded a Royal Commission of 1851 Research Fellowship for 2020! Alex will use his fellowship to investigate catalysts and polymerization processes to make new polymers and materials with a focus on high performance sustainable materials.

- Congratulations to Alex who has been awarded a Royal Commission of 1851 Research Fellowship for 2020! Alex will use his fellowship to investigate catalysts and polymerization processes to make new polymers and materials with a focus on high performance sustainable materials.
- Congratulations Dr Nattawut (Peck) Yuntawattana on completing his PhD viva, September 2020
- Congratulations to Peck on a successful DPhil viva for this thesis. With thanks to the Royal Thai Government Scholarship which supported his studies in Oxford. We wish him all the best on his immediate return to Thailand and for his subsequent postdoctoral studies in the USA.

- Congratulations to Peck on a successful DPhil viva for this thesis. With thanks to the Royal Thai Government Scholarship which supported his studies in Oxford. We wish him all the best on his immediate return to Thailand and for his subsequent postdoctoral studies in the USA.
- Congratulations PhD student Gloria Rosetto for receiving the Student Union Award "Sustainable Advocate"
-
With the most nominations of anyone, this beloved E&E rep has a true record of advocating for sustainable living, during lockdown no less!
-
- Congratulations to PhD Student Wouter Lindeboom for being awarded the Leathersellers' Company Scholarship, starting in October 2020 from St Catherine College

- Congratulations to 2020 Part II Students Emma Moreby and Nathan Zhang on both achieving First Class MChem Degrees! Emma was also awarded the Inorganic Chemistry Part II Thesis Prize – Congratulations Emma, fantastic news.
- Congratulations to our students Nathan Zhang and Emma Moreby for successfully completing and handing in their part II theses. Great effort in these difficult times and we wish you both every success in your future careers!
- June 2020


- June 2020
- Science to Enable Sustainable Plastic , June 2020
- The worst environmental impacts of plastics are a common sight in the news or on social media – but they also fulfil vital roles in society, and in some cases can even be a more sustainable option compared to alternatives. But more research is urgently needed in order to make them fit for the future. In November 2019, Charlotte Williams chaired a group of researchers, representative of learned societies, and funders from China, Germany, Japan and the UK at the Chemical Sciences and Society Summit (CS3). Over the course of a three day summit, they developed a plan for how to create a circular economy for plastics, preserving the crucial function they serve in society while introducing much better recyclability and reusability into their design. Today we’re announcing publication of the white paper, which outlines four major research challenges – areas in urgent need of development.
- Read the report now
-
Forbes article about the report including an interview with Charlotte Williams

- Chemistry World: Charlotte has written an article for Chemistry World’s special issue on Plastics: https://www.chemistryworld.com/opinion/addressing-the-plastics-problem/4...
-
May 2020

-
- Congratulations to Tom Chen on completing his PhD viva - Dr Chen, April 2020

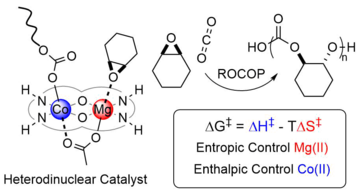
- Congratulations to Arron on the publication of his work on heterodinuclear catalysts for carbon dioxide and epoxide copolymerization in Nature Chemistry. The paper describes a highly active and selective Co(II)Mg(II) catalyst which shows remarkably enhanced performance compared to any homodinuclear analogue. Through detailed investigation of the polymerization kinetics, the factors influencing the synergy in this catalyst are also explored, with the Co(II) being responsible for reducing the transition state enthalpy and the Mg(II) reducing the transition state entropy compared to homodinuclear analogue catalysts. This rare insight into how heterodinuclear synergy or cooperativity enhances performances may be more generally applicable and could be a new design feature to exploit in preparing polymerization catalysts.
- Oxford Martin School Lecture by Charlotte Williams, February 2020
- Thanks to the Oxford Martin School for hosting and chairing Charlotte’s public lecture “Future Options for Making Plastics More Sustainable.” It was inspiring and interesting to hear the range of audience views and to discuss some of the technical options available now and in future to improve plastics’ sustainability.

- Thanks to the Oxford Martin School for hosting and chairing Charlotte’s public lecture “Future Options for Making Plastics More Sustainable.” It was inspiring and interesting to hear the range of audience views and to discuss some of the technical options available now and in future to improve plastics’ sustainability.
-
Macro Group UK Award
-
Charlotte Williams and Sebastian Perrier (Warwick University) are jointly awarded the Macro Group UK Medal. Hugo Bronstein (Cambridge University) wins the Macro Group UK Young Researcher Medal (Hugo is a former Williams group PhD student). Dr John Tobin and Dr Pratik Gurnani are jointly awarded the John Weaver PhD Prize. Congratulations to all the prize winners and thanks to the Macro Group UK committee for organizing a very interesting symposium at Warwick University.
-
November 2019

-
- Chemical Sciences and Society Summit: Charlotte is chairing the 8th Chemical Sciences and Society Summit (CS3) Science to Enable Sustainable Plastics at the Royal Society of Chemistry in London.
- November 2019

- November 2019
- Nature: A recent publication by an international team, including Charlotte Williams, in Nature highlights the potential and future challenges in scaling carbon dioxide utilization across a range of agricultural and industrial sectors.” https://www.nature.com/articles/s41586-019-1681-6
- November 2019
- Periodic: Our research was featured on Oxford Chemistry's annual magazine called Periodic.
-
October 2019
-
- Plastics Transformation Map: Partnership with the World Economic Forum through the Future of Plastic program at the Oxford Martin School to create a Plastics Transformation Map, in which the key issues related to plastics are addressed. It also shows the interconnectedness of the problem by relating it to other world issues.
-
September 2019
-
- EPSRC Fellowship: Awarded a 5-year fellowship to investigate how to make the production of plastics more environmentally friendly.
- September 2019
- Royal Society Policy Briefing: Sustainable synthetic carbon-based fuels for transport, part of the steering group behind the policy briefing.
- September 2019
-
Chemical Science Editor's Choice: "Heterodinuclear zinc and magnesium catalysts for epoxide/CO2 ring opening copolymerizations" was included in the selection of papers which represent outstanding contributions published in (bio)inorganic chemistry, catalysis and spectroscopy, chosen by editor Serena DeBeer.
-
August 2019
-
- Nature Communications Editor's Highlights: “Easy access to oxygenated block polymers via switchable catalysis” selected by editor P. Saini in the Organic Chemistry and Chemical Biology section.
- July 2019
- Chemistry World Article: Carbon dioxide generated then recycled in polymerisation bicycle
- June 2019

- June 2019
- Congratulations to Prof. Charlotte Williams for being awarded the 2018 Macro Group UK Medal!
- Congratulations to Tim Stößer on completing his DPhil viva, November 2018

-
Congratulations to Dan Mulryan for being awarded the Part II Thesis Prize in Inorganic Chemistry, October 2018

- Congratulations to Alice Leung on completing her DPhil viva - Dr Leung (October 2018)

-
International Coordination Chemistry Conference (ICCC2018), Sendai, Japan (August 2018)
-
Arron Deacy - Highly Active Magnesium-Cobalt Catalyst for the ROCOP of CO2-CHO
-
Congratulations to Arron for being awarded the ICCC2018 Poster Prize!

-
- Congratulations to Ni Yi on completing her DPhil Viva - Dr. Yi (June 2018)

- Congratulations to Gemma Trott on completing her DPhil Viva - Dr. Trott! (May 2018)

- Faraday Discussion: Designing Nanoparticle Systems for Catalysis, London (May 2018)
- Saïd Saïd - Organometallic Route to Exfoliated Layered Zinc Hydroxides (LZHs) (Poster)
- Congratulations Said for being awarded The Faraday Discussions Poster Prize!

- New Scientist Article: From Pollution to Solution
-
17th March 2018, issue 3169.

-
- Congratulations to Prof. Charlotte Williams, awarded the 2018 Otto Roelen Medal!
- Charlotte Williams is the recipient of the DeChema Otto Roelen Medal for 2018. It is awarded by DECHEMA and the German Catalysis society every two years for outstanding scientific work in the field of catalysis, the award is sponsored by OXEA GmbH. She was awarded the Medal at the 51st German Catalysis Meeting, held in Weimar from 14-16 March 2018.
- Congratulations Charlotte Coleman on completing her DPhil Viva - Dr. Coleman! (February)

- UK Catalysis Conference (UKCC2018), Loughborough (January 2018)
- Tim Stößer - Selective Polymerization Catalysis: Using Metal - Salen Catalysts to Selectively Polymerize from Mixtures of Monomers (Keynote)
- Alice Leung - Colloidal Cu/ZnO Nanocatalysts for the Hydrogenation of CO2 to Methanol: The Influence of the Synthetic Route (Oral)
- Arron Deacy* - Heterodinuclear Catalysts (LZnMXn) For CO2-CHO Copolymerisation (Poster)
- Tom Chen - Multiblock Copolymers From 4 Monomer Mixture Using a ‘Switchable’ Catalyst (Poster)
- Nattawut Yuntawattana - Phosphasalen Indium Ring Opening Polymerisation Catalysts: High Iso-selectivity to Produce Stereoblock PLA (Poster)
- Dr. Sumesh Raman - Recycling CO2 in Polymer Synthesis: Selective Catalysis Using O-Carboxyanhydrides and Epoxides to Make Poly(ester-b-carbonates) (Poster)
(*Winner of the UKCC2018 Poster Prize)
-
Congratulations to Prof. Charlotte Williams, awarded the Sir John Meurig Thomas Catalysis Medal 2017!
-
Charlotte Williams has won the Sir John Meurig Thomas Catalysis Medal for outstanding achievement in catalysis for multidisciplinary contributions to the development and applications of catalysts for sustainable chemistry. She was awarded the medal at the UK Catalysis Hub Winter Conference on the 14th of Decembe r 2017. The award is made by the UK Catalysis Hub to recognise the achievements of a mid-career scientist working in catalysis in the United Kingdom.
-

-
-
Frontiers in Green Materials, London (December 2017)
-
Tim Stößer - Block Copolyesters from Mixed Monomer Feedstocks: A One-Pot Combination of ROCOP and ROP (Oral)
-
Dr. Yunqing Zhu - Semi-Renewable Multi-block Polyesters Demonstrating High Elasticity and Shape Memory Effects (Poster)
-
Ni Yi - (Poster)
-
Tom Chen - Semi-Renewable Multiblock Copolymers from Monomer Mixtures using a ‘Switchable’ Catalyst (Poster)
-
- Chemistry World Article : CO2 recycling - An Uphill Struggle (27th October 2017)

- BBC News - From Fluffy Pillows To Concrete: The Uses Of Captured CO2 - Econic Technologies (October 2017)
- International Symposium on Ionic Polymerization, Durham (September 2017)
- Tim Stößer - Block Copolyesters from Mixed Monomer Feedstocks: A One-Pot Combination of ROCOP and ROP (Poster)
- Dalton Young Members Event (DYME), Bath (September 2017)
- Dr. Christopher B. Durr - O-Vanillin Derived Titanium Phenoxyimine Initiators for Ring Opening Polymerisation of Macrolactones (Oral)
- Arnaud Thevenon - Indium Phosphasalen Catalysts for Low CO2 Pressure/Epoxide Copolymerisation: Evidence of an Unexpected Mononuclear Pathway? (Oral)
- Alice Leung - Layered Zinc Hydroxide Monolayers by Hydrolysis of Organozincs (Oral)
- Charlie Coleman - Asymmetric hybrid salen/phosphasalen group 13 initiators for the iso-selective ring-opening polymerisation of rac-lactide (Poster)
- Gemma Trott - Synergy using Zinc and Magnesium: Heterodinuclear polymerisation catalysis showing enhanced performances (Poster)
- Nattawut Yuntawattana - Indium Phosphasalen Catalysts: Synthesis, Characterization and High iso-selective in ROP of rac-lactide (Poster)
-
BASF International Summer Course, Ludwigshafen (August 2017)
-
Tim Stößer - Selective Synthesis of Block Copolyesters from Mixed Monomer Feedstocks (Poster)
-
- The Times Newspaper - Feature Article - Econic Technologies (August 2017)

Image - The Times Online
- EPSRC - PIONEER - Feature Article - Charlotte Williams

- International Symposium on Macrocyclic and Supramolecular Chemistry, Cambridge (July 2017)
- Arnaud Thevenon - Switchable Dizinc Macrocycle Catalysts: From Highly Active Lactide Polymerization to Block Copolymers (Poster)
- Gemma Trott - Synergy using Zinc and Magnesium: Heterodinuclear polymerization catalysis showing enhanced performances (Poster)
- Macro Group YRM, Edinburgh (June 2017)
- Tim Stößer* - Selective Synthesis of Block Copolyesters From Mixed Monomer Feedstocks (Poster)
(*Winner of the Macro Group YRM Poster Prize)
- 100th Canadian Chemistry Conference, Toronto, (May 2017)
- Gemma Trott - Synergy using Zinc and Magnesium Heterodinuclear Polymerization Catalysis Showing Enhanced Performances (Oral)
- Royal Society Discussion Meeting - Providing sustainable catalytic solutions for a rapidly changing world (May 2017)
- Prof. Charlotte Williams - Catalysts control to allow selective polymerizations from mixtures: a useful strategy to deliver future materials
- Dr. Jennifer Garden - Synergic heterodinuclear catalysts: applications in CO2/epoxide copolymerisation (poster)


- 253rd ACS National Meeting & Exposition - San Francisco, USA (April 2017)
- Prof. Charlotte Williams
- Switchable polymerization catalysts: Selective block copolymers from monomer mixtures (oral)
- Greater than the sum of their parts: Heterodinuclear polymerization catalysts (oral, ACS Award for Distinguished Service in the Advancement of Inorganic Chemistry: Symposium in honor of William B. Tolman)
- Charlie Coleman - Asymmetric hybrid salen/phosphasalen initiators for the iso-selective ring-opening polymerisation of rac-lactide (oral)
- Alice Leung - Organometallic route to layered zinc hydroxides and their exfoliated monolayers in apolar solvents (oral)
- Arnaud Thevenon - Dizinc lactide polymerization catalysts: Hyperactivity by control of ligand conformation and metallic cooperativity (oral) & Switchable di-zinc macrocycle catalysts: From highly active lactide polymerization to block copolymers (poster)
- Professor Williams receives the RSC Corday-Morgan Prize (2016)
- ''Awarded for her work on polymer chemistry and catalysis and in particular recognition for her contributions to the catalytic activation of renewable resources to make polymers and fuels''
- BBC Radio 4 Costing the Earth - Putting the Fizz Back into Planet Earth (2016)
- Congratulations to the following previous post docs of the group, we are all very sad to see you leave and we wish you all the best for your future endeavours! (2016)
- Dr. Jennifer Garden (Christina Miller Research Fellow, University of Edinburgh)
- Dr. Sebastian Pike (Herchel Smith Research Fellow, University of Cambridge)
- Dr. Charles Romain (Junior Research Fellow, Imperial College London)
- Congratulations to Gemma Trott for winning the Sir Geoffrey Wilkinson Dalton Poster competition at Dalton 2016, Warwick!

- Professor Williams receives WISE award for eco-plastics startup (2015)
- Williams group take part in Imperial festival (2015)


